|
|
TITLE: |
||
|
|
|||
|
DOCUMENT ID: |
5400 Appendix T2 Forms and Schedules |
||
|
|
|||
This
appendix contains certain forms and content listings to assist files custodians
in managing Environmental, Safety, Health, and Quality (ESH&Q)-related
records. Refer to the Jefferson
Lab Records Management Handbook for assistance in knowing how, what, and
when to use these forms.
Many
activities previously completed with paper forms can now take place using
online submission and request forms at http://www.jlab.org/div_dept/cio/IR/records/index.html.
ES&H Manual Chapter
5400 Appendix T1 Summary of ES&H Documentation Requirements contains information regarding
most of the ESH&Q-related regulatory and contractual documentation and
recordkeeping requirements at Jefferson Lab.
·
Figure 1: Records Inventory and Disposition Schedule (RIDS)
This is the Department of Energy
(DOE) form 1324.10 (a sample form follows) that is used to maintain the records
inventory and identify disposition authority of all records maintained by a files
custodian. In general, this form is used when a record file system is
initially established. This is accomplished by the files custodian
in coordination with the Jefferson Lab Records Manager.
The RIDS should be used to
maintain information of all ESH&Q-related records for which you are
responsible, including records in storage. The information contained on this
form is a summary of existing files, and how and when they can be appropriately
disposed of. Using this form simplifies the review process when it comes time
to dispose of old records.
o An example -Environment
Management Files. The disposition authority is DOE Records Schedule (DOERS)
which determines the authorized disposition of the record. The disposition authority
is cited at DOERS 1.(2) for these particular records,
which states to destroy when 15 years old.
·
Figure 2: Records Disposition and Retrieval Request (RDR) Form
This form is used for all
document transactions between a files
custodian and the Records Manager.
This form must be submitted to
the Records Manager whenever a files
custodian wishes to place or retrieve a file from the records storage room.
Using this form will help to track all record transactions, ensuring that
documents do not become misplaced.
o
An
example -Radiation Control Exposure Records. Since these files must be kept for
an extended period of time (75 years), it may be beneficial to transfer these
files to the records storage room. Complete the RDR and submit it, along with
the files to be stored, to the Records Manager.
This
form is used by the divisions/departments to maintain a record of the storage
and disposition of records, including ESH&Q-related information. This form
should be used when maintaining internal records within the office, as well as
to keep track of records that have been sent to the records storage area.
The
Records Log Form contains information on the storage location of your files, as
well as a description of the record and disposal date.
- An example of the use of the Records Log Form
is the management of accident and injury records. As these records must
be kept for five years, they will need to be filed away. By noting their
location on the Records Log Form, retrieval when needed will be made
easier. The Records Log Form will also tell you any actions, such as
disposal, that have been taken with these records.
This
Records Schedule is acceptable guidance for determining retention requirements,
and should be used when conducting your inventory. The table of contents is
included in Table 1.
o
Schedules
that may be of use include Schedule I which contains information for Civilian
Personnel Records, including medical records and personal injury records.
- Request for Records Disposition Authorization
This
form (not included) may be used to request a disposition schedule from the DOE
when records at hand do not appropriately fit into established schedules. This
form is typically used by the Records Manager for correspondence with the DOE,
and will seldom be used by other staff.
For
further assistance with these forms or documents, contact the Jefferson Lab
Records Manager at ext. 7805.
Figure 1:
Records Inventory and Disposition Schedule (RIDS)

Figure 2:
Records Disposition and Retrieval Request (RDR) Form

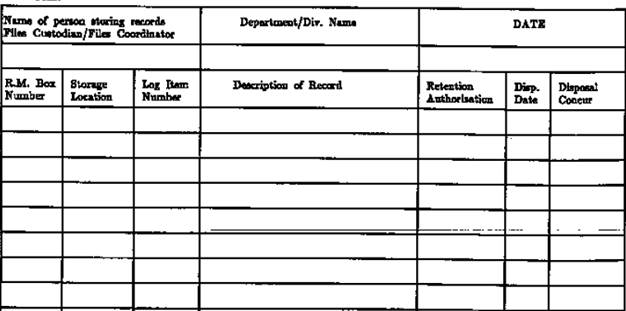
Table 1: Jefferson Lab Records
Schedules
Table of Contents
|
Schedule |
I. |
Human
Resources |
|
Schedule |
II. |
Business Services (Procurement and
Finance) |
|
Schedule |
III. |
Environment, Health, Safety, and
Quality/Occupational Medicine |
|
Schedule |
IV |
Facility/Facilities Management |
|
Schedule |
V. |
Project Costs, Inventory and
Accounting |
|
Schedule |
VI. |
Research and Development (Physics and
Accelerator) Project Records |
|
Schedule |
VII. |
R&D Program Management Records |
|
Schedule |
VIII. |
R&D Collaborations and Technology
Transfer |
|
Schedule |
IX. |
Legal, Auditing, and Administration |
|
Schedule |
X. |
Director/Public Relations |

1.0
Revision
Summary
Revision 0.1 – 08/13/14 – Periodic
Review – Updated
TechPOC from S.Smith to T.Johnson
|
ISSUING
AUTHORITY |
TECHNICAL
POINT-OF-CONTACT |
APPROVAL
DATE |
REVIEW DATE |
REV. |
|
ESH&Q Division |
08/13/14 |
08/13/17 |
0.1 |