A |
|
|
Approval/Approved |
The dated signature that denotes acceptance by an authorized individual. (This can be an electronic format.) |
B |
|
|
|
|
C |
|
|
Cause Analysis |
Performed to determine the root cause for each criterion found non-compliant with requirements. |
|
Contributing Cause |
An event or condition that either caused the occurrence under investigation or contributed to the unwanted result. If it were not for this event or condition, the unwanted result would not have occurred or would have been less severe. |
|
Controlled Document |
The minimum requirements for a document to be considered controlled include periodic review and approval (typically every three years), and retention of revisions. |
|
Corrective Action |
Activity(ies) that, when completed, restores an issue, (e.g. service, item, component, or process) to a state of compliance with specifications, procedures, or regulatory requirements. Activity(ies) that, when completed, prevent or minimize the probability or severity of a recurrence by addressing the causal factors of the event. |
|
Criteria/Criterion |
A single principal or standard expressed within a requirement document. |
|
Criteria Review and Approach Documents (CRAD) |
Any document that defines the necessary criteria for a program or process to be considered adequate. |
D |
|
|
Document or Documented |
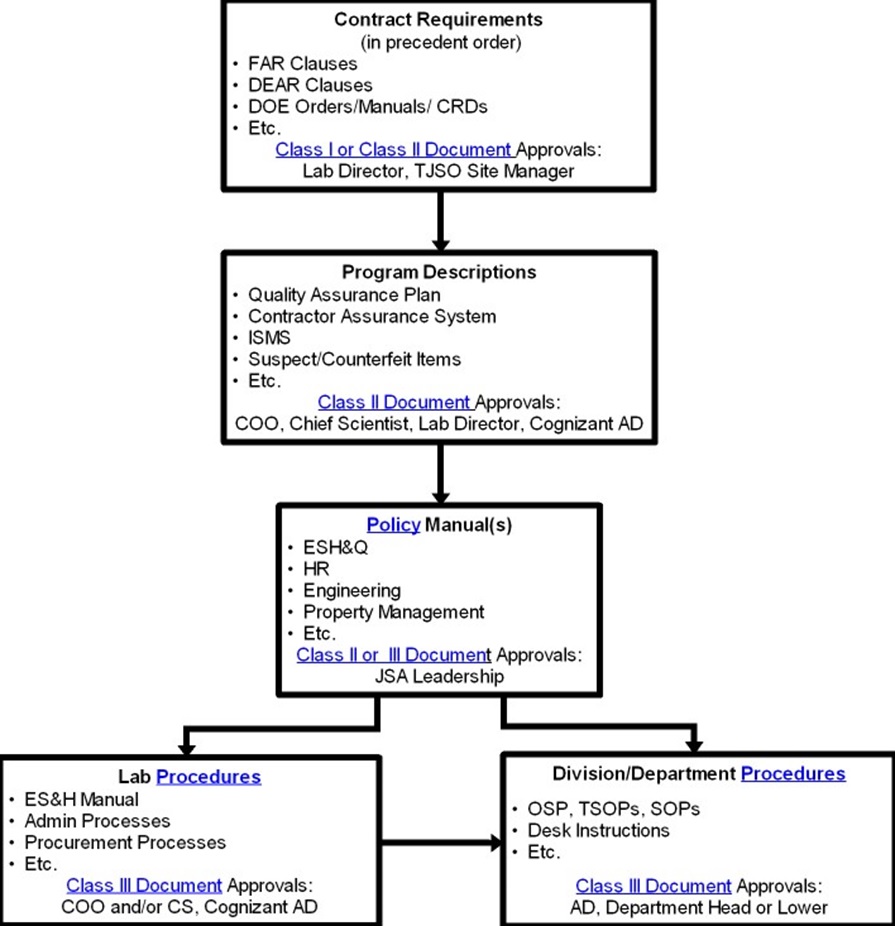
The current version in a supported medium, e.g. paper, magnetic, electronic or optical computer disc, photograph or master sample, or any combination thereof. Jefferson Lab utilizes three separate categories of controlled documents:
|
|
Document Hierarchy |
|
E |
|
| Effective | the ability of a program, or its components, to accomplish the specified objective(s). |
|
Established |
Physical proof that something has existed since it was required (minus a reasonable period needed to initiate). |
|
Event |
1. An Assessment/Audit performed on the behalf or request of an inside/outside agency or entity, or as part of a contract commitment. Generally assessments/audits require a formal report to document any noted deficiencies. (Includes: ES&H Manual Revisions, Environmental Management System (EMS) Reviews, Independent Assessments (IA), Management-Self (or similar) Assessments (MSA), Project Deadlines, Safety Team Reviews, and Worker Safety Committee findings) |
F |
|
|
Finding |
An adverse event, or a discovered non-compliance with a requirement. |
G |
|
|
Graded Approach |
A method used that will determine the appropriate level of analysis, management controls, documentation, or other necessary action(s) to decide where and when resources are to be allocated to ensure items and/or processes have the greatest effect upon personnel, environment, safety, health, cost, data, equipment, performance, quality and schedule. (See Performance Assurance Department Graded Approach Procedure) |
H |
|
|
|
|
I |
|
|
Implemented |
Documented proof that a process, program, etc. has been effectively and consistently introduced. Including dates of occurrences names of individuals who perform the activities, and documented processes/procedures. |
|
Initiate |
The period determined reasonable to start a new program or process. This would include documenting original procedures and training workers. |
|
Inspection |
A detailed physical examination using a standard procedure. |
|
Issue |
A process deficiency; regulatory non-compliance; procedure inadequacy; material or equipment deficiency; identified during day-to-day work or by a formal review process. Examples include, but are not limited to, an item, service, part, component, or process that is not functioning correctly (out of compliance or not in accordance with applicable specifications); physical defects; test failures; incorrect or incomplete documentation; and deviations from prescribed instructions, procedures, or drawings, etc. (Observations or opportunities-for-improvement, provided within a formal event document, that are not deviations from requirements, may be considered issues based on Management discretion). |
J |
|
|
|
|
K |
|
|
|
|
L |
|
|
Line of Inquiry (LOI) |
An objective question, item, or guidance, used to determine if a criterion’s objective is achieved. A single action required to confirm compliance with a criterion. |
|
Latent Organizational Weakness (LOW) |
An undetected deficiency in organizational processes and values that create workplace conditions that provoke error or degrade the integrity of defenses. |
M |
|
|
Maintained |
Evidence of regular review, inspection, and updates, using a documented standard process. |
N |
|
|
Noteworthy Practice |
A positive aspects of a program that could be used as a model for others. |
O |
|
|
Opportunity for Improvement (OFI) |
A deviation from best management practices, isolated or minor deviation from procedural requirements, or recommendation(s) that, if corrected, would contribute to continuous improvement goals. A Line of Inquiry (LOI) that is addressed, but determined to be less than adequate. |
P |
|
|
Policy |
An overarching statement that summarizes the principle of an organization, or program. |
|
Procedure |
Directions used to achieve a single goal. A series of action steps and the individual(s) responsible for performance. |
|
Process |
Several procedures used to achieve an objective. |
Q |
|
|
|
|
R |
|
|
Record |
A collection of archived documents (e.g. past revisions of documents), which are no longer subject to revision. |
|
Regulation |
A document called out within a requirement that defines the necessary criteria to ensure a program or process is adequate. |
|
Requirement |
A document specifically called out within the JSA/DOE Contract No. DE-AC05-06OR23177. They include, but are not limited to: DOE orders, state, and local laws and permits, and define the necessary criteria to ensure a program or process is adequate. |
|
Review |
An examination to determine accuracy, generally on a particular subject. Direction/comment/acceptance is given to the owner to achieve adequacy. Reliance of overall accuracy of the item is the owner’s responsibility. |
|
Root Cause |
The causal factor(s) that, if corrected, would prevent recurrence of the occurrence. It is the most basic cause that explains why the event happened, that can reasonably be identified, that senior management has the control to fix, and for which effective recommendations for corrective actions to remedy the problem, prevent specific recurrence of the problem, and preclude occurrence of similar problems can be generated, if necessary. (See DOE STD-1197-2011) |
S |
|
|
|
|
T |
|
|
|
|
U |
|
|
|
|
V |
|
|
|
|
W |
|
|
|
|
X |
|
|
|
|
Y |
|
|
|
|
Z |
|
|
|
|
|
Criteria Review and Approach Documents |
|
|
Line of Inquiry |
|
|
LOW |
Latent Organizational Weakness |
|
Opportunity for Improvement |
|
|
TJNAF |
Thomas Jefferson National Accelerator Facility |
|
TJSO |
Thomas Jefferson Site Office |
|
ISSUING AUTHORITY |
TECHNICAL POINT OF CONTACT |
APPROVAL DATE |
REVIEW DATE |
REV. |
|
Performance Assurance |
N/A |
N/A |
N/A |